Case Study
Development of a device for laser marking of implant nails
Clearly labelled – for the life of the product
Category: Turnkey Solution
Client: world leader in medical technology
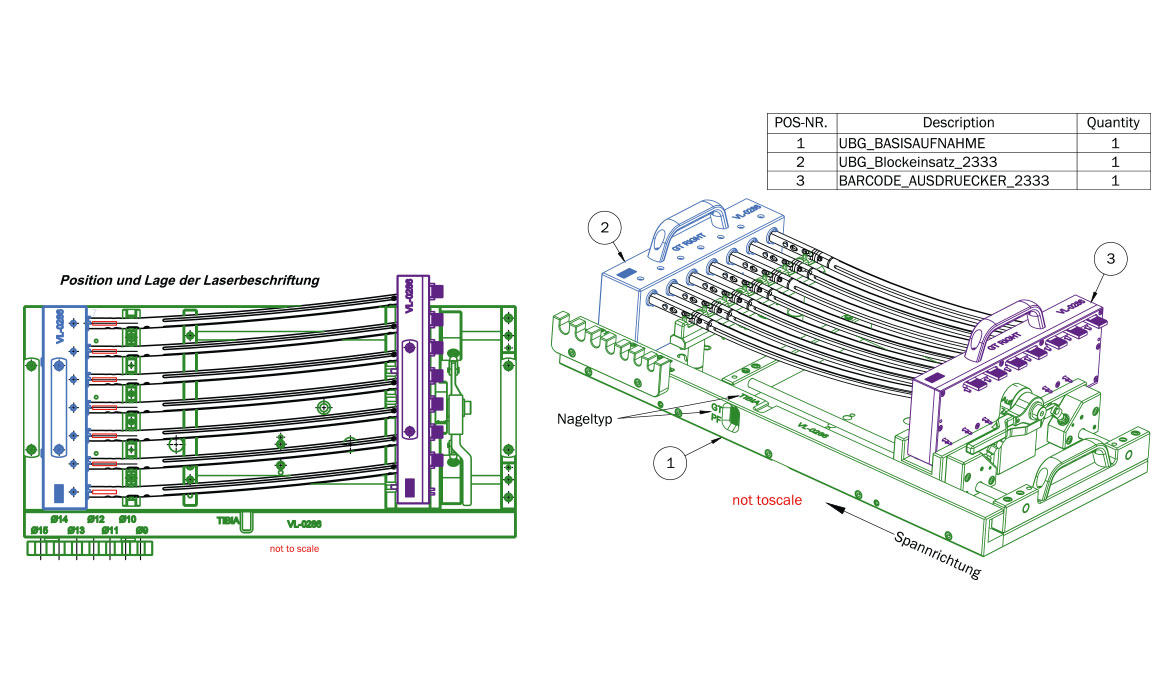
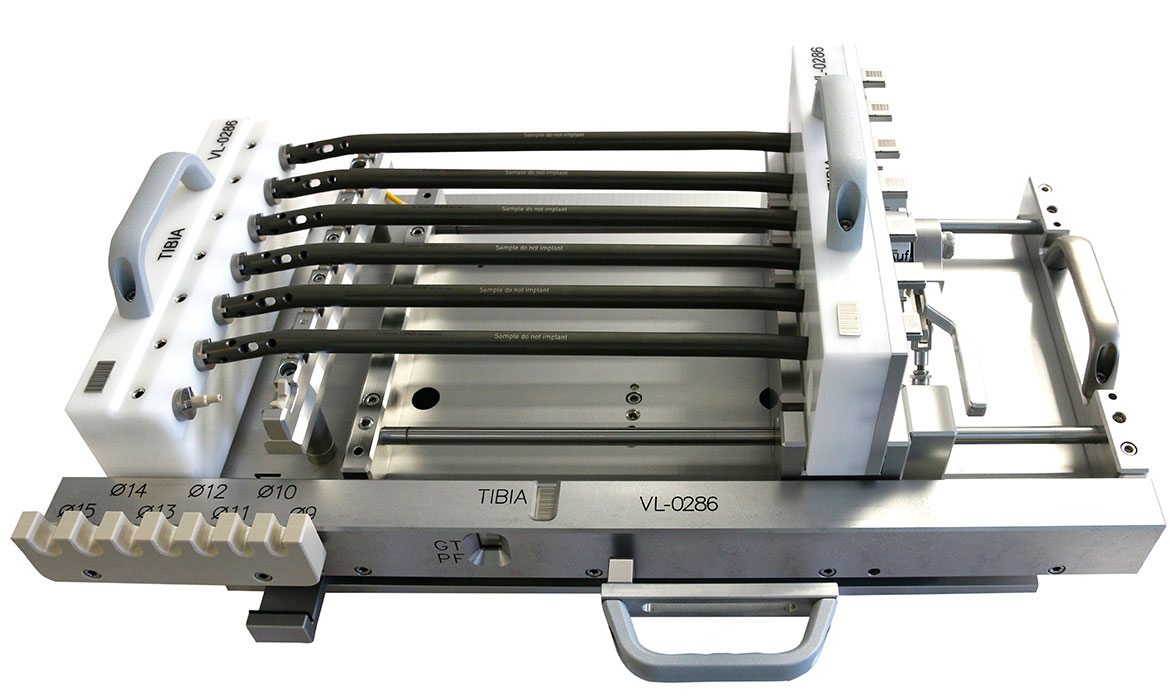
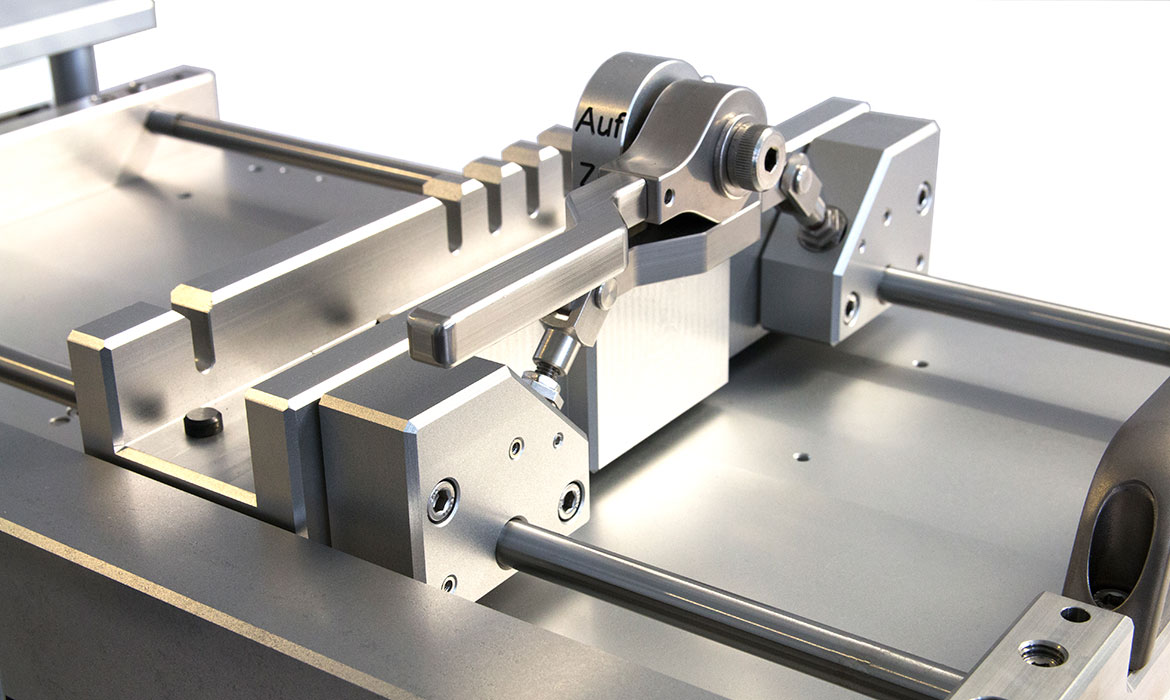
On behalf of a manufacturer of innovative products for orthopaedics, medicine and surgery, neurotechnology and spinal surgery, a customised device was developed for holding certain intramedullary nail implants for laser marking. Taking into account the poka yoke (error prevention) principle, it was necessary to prevent medullary nail implants from being incorrectly marked. In addition to the actual development of the device, the scope of services includes production, logistics and commissioning.
Client benefit
From the original requirement to process five nails simultaneously, the device could be expanded to seven during development. At the same time, the device can also be operated with fewer nails. The device was designed and manufactured without any technical complications.